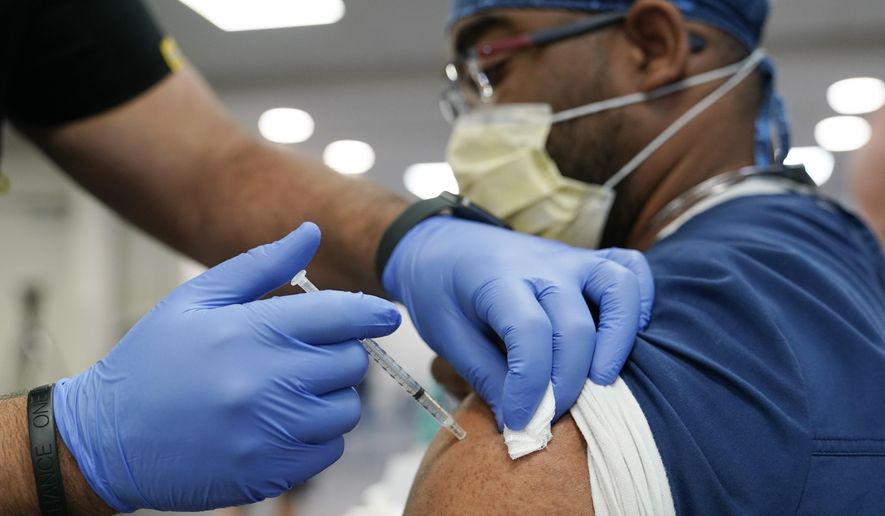
Advisers to the Centers for Disease Control and Prevention endorsed a booster plan Thursday for all three COVID-19 vaccines, nudging forward a strategy to let Americans mix and match brands to bolster their defenses and avoid rare, though severe, side effects linked to vaccine technologies deployed in the U.S.
The CDC Advisory Committee on Immunization Practices voted 15-0 to approve a half-dose booster of the Moderna vaccine alongside a previously approved full-dose Pfizer booster.
They supported an extra dose of the one-shot Johnson & Johnson version by the same margin.
Any adult who received the J&J version at least two months ago is eligible for a boost because of concerns its efficacy lags behind the other versions.
People who got their second dose of the Pfizer or Moderna version at least six months ago can come forward if they are 65 or older, an adult with an underlying health condition or a worker whose job could expose them to the virus.
CDC Director Rochelle Walensky accepted the advisers’ recommendations and touted the range of booster choices.
“Eligible individuals may choose which vaccine they receive as a booster dose,” an agency statement said. “Some people may have a preference for the vaccine type that they originally received and others, may prefer to get a different booster. CDC’s recommendations now allow for this type of mix and match dosing for booster shots.”
Millions more will join the 11.6 million Americans who have received Pfizer boosters. They will start comparing vaccines instead of taking what they can get after the Food and Drug Administration this week approved a mix-and-match strategy that lets anyone eligible for a booster choose from the trinity of vaccines.
A chunk of the 15 million Americans who received the one-shot Johnson & Johnson version might switch to another vaccine instead of accepting another J&J dose. Studies showed a more significant antibody jump in people who got the Moderna and Pfizer version as a booster.
“Vaccinators would love the flexibility with mixing boosters. And yes, I believe that recipients of one vaccine are asking for a booster with the other, especially those who are more literate with the science behind what we call heterologous boosting,” Litjen Tan, the chief strategy officer at the Immunization Action Coalition, told The Washington Times, using the technical term for switching.
Dr. Tan said there might be challenges. Record-keeping will be essential to see how mixing and matching works and whether people have adverse reactions.
Some people might not know which brand to pick. Dr. Tan said limited inventory at specific sites might reduce patient confusion.
“The vaccinator will only need to boost with the vaccine [she or he] has on hand,” he said.
Another upside to vaccine switching emerged during Thursday’s discussion. Young men and women could avert side effects by crossing over to a vaccine that was manufactured differently.
CDC officials said there have been 47 confirmed reports of a rare blood clotting problem, known as thrombocytopenia syndrome (TTS), in the U.S. out of 15.3 million recipients of the one-shot J&J vaccine, which is produced by the drugmaker’s Janssen division. Five deaths have been reported as a result of TTS, which tends to affect women younger than 50.
Dr. Pablo J. Sanchez, a pediatrics professor at Ohio State University and an advisory committee member, said he thinks J&J recipients need boosters, but he is “very, very concerned” about the idea of a second J&J dose given the blood-clotting risk. The side effect also has been documented with the AstraZeneca version in Europe. Both use an inactivated, or adenovirus, platform.
Meanwhile, there are concerns about inflammation of the heart muscle, or myocarditis, in young males who receive the Pfizer or Moderna vaccines, which use messenger RNA to teach the body to detect and fight the virus.
Rates of the condition, which tends to be mild and occur after the second dose, are as high as 100 cases per 100 million doses in males younger than 20.
Some advisers said mixing and matching might allow recipients to cross over to a safer option.
“I worry about young women and TTS with the Janssen, and I flip it over and worry about myocarditis and young men,” said Dr. Helen Keipp Talbot, an associate professor of medicine at Vanderbilt University in Nashville, Tennessee. “I think the opportunities for these heterologous boosts are priceless. It gives those who received Janssen — if you’re a young woman, to receive a messenger RNA. And if you’re a young man who’s received the messenger RNA, maybe you switch over to the Janssen. We’re in a different place in the pandemic than we were earlier. Vaccines are readily available.”
The debate about boosters has emerged with concern about waning immunity from the initial vaccination series.
The Biden administration made a big push for boosters in August as the delta variant of the coronavirus struck and reports of breakthrough infections in the vaccinated spiked. Skeptical scientists said the shots performed their primary function of preventing many hospitalizations and deaths.
CDC advisers said the data about boosters for mRNA vaccines remains thin, but they agreed that J&J recipients could use another dose to boost efficacy.
Biden administration officials say they are pushing boosters because they are worried about what might lie ahead and want to be prepared. Regulators might adjust the range of people eligible for boosters in the coming weeks.
“It’s a very dynamic situation. We’re watching it extremely carefully, and we’ll take appropriate action to protect the public should it be necessary,” acting FDA Commissioner Janet Woodcock told reporters Wednesday.
Dr. Woodcock’s regulators authorized requests from Moderna and J&J to join Pfizer in offering boosters. It also approved the mix-and-match plan, kicking the concept to the CDC to determine how the strategy should be implemented.
Penny Heaton, the head of global therapeutics for J&J’s Janssen division, focused on the potential benefits of boosting with another J&J shot in her Advisory Committee on Immunization Practices presentation. Protection against symptomatic COVID-19 is 94% in the U.S., up from 74% after a single shot in an earlier study.
FDA officials have said pharmacists and vaccinators will rely on recipients’ word in attesting that they qualify for a booster but may use questionnaires to determine eligibility.
Dr. Walensky is expected to accept the advisory committee’s recommendations in issuing guidance to states, though she did break from the panel by adding front-line workers to a recent booster approval for Pfizer.
• Tom Howell Jr. can be reached at thowell@washingtontimes.com.



Please read our comment policy before commenting.