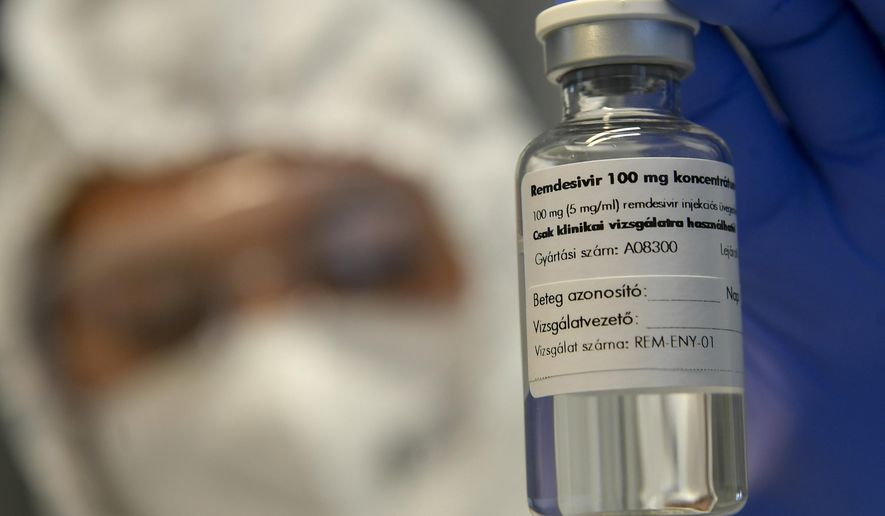
The Food and Drug Administration on Thursday issued emergency approval for a rheumatoid arthritis drug in combination with the antiviral medication remdesivir as a treatment for hospitalized adults and children with COVID-19.
Baricitinib combined with remdesivir reduced recovery time of hospitalized patients within 29 days after initial treatment compared to patients who received a placebo with the antiviral drug, the FDA said, citing the results of a clinical trial.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Dr. Patrizia Cavazzoni, acting director of the FDA’s Center for Drug Evaluation and Research. “Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients.”
The trial, conducted by the National Institute of Allergy and Infectious Diseases, included 1,033 patients with moderate to severe COVID-19. Researchers gave 515 patients baricitinib plus remdesivir while the other 518 received a placebo plus the antiviral drug.
The median time for recovery from COVID-19 was seven days for baricitinib plus remdesivir and eight days for the placebo and remdesivir. The chances of a patient dying or requiring ventilation at day 29 was lower for those who received baricitinib than those who were given a placebo. Patients who received baricitinib also had a greater chance of showing “clinical improvement” at day 15 compared to those who took a placebo.
The FDA granted the emergency use authorization to Eli Lilly and Company.
• Shen Wu Tan can be reached at stan@washingtontimes.com.



Please read our comment policy before commenting.