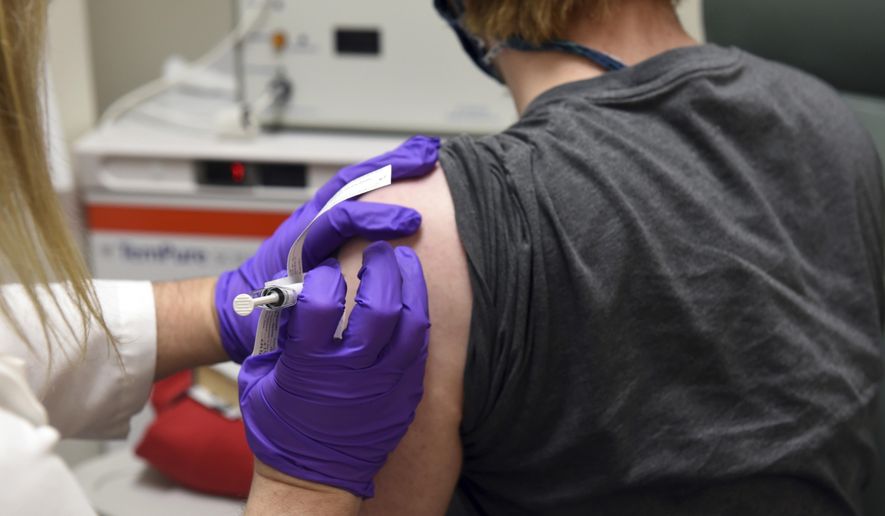
Two drugmakers in the race for a COVID-19 vaccine are on track for emergency approval before the end of the year, the Trump administration said Wednesday, with Pfizer reporting its shots were 95% effective in a final analysis and Moderna set to follow close behind.
Pfizer, of New York, said it counted 170 cases within its 43,000-member trial and that all but eight of the infections were in the placebo group. It plans to request authorization from the Food and Drug Administration “within days,” after the shots were effective across age, gender, race and ethnicity groups.
The results are an improvement over prior data that showed 90% efficacy and on par with initial results from Moderna, of Massachusetts, which will probably count enough cases to report final efficacy results from its own messenger-RNA vaccine soon, given widespread transmission of the coronavirus.
“It is very likely Moderna will shortly be obtaining that number and announcing it,” said Moncef Slaoui, who is overseeing vaccine development for the White House.
The administration said it expects the FDA to grant emergency-use authorization to both vaccines by next month, putting them in a position to distribute 40 million doses — enough to vaccine 20 million people with the two-dose vaccines — before the new year.
Army Gen. Gustave Perna, a logistics expert in charge of President Trump’s “Operation Warp Speed,” said shots will go out the door within 24 hours of the FDA giving the green light. He said 64 jurisdictions — the 50 states, eight territories and six metropolitan cities — will tell his operation where to send the vaccines, which will be distributed based on population.
“We implement their plan,” Gen. Perna said.
The U.S. and world desperately need a vaccine to bring the coronavirus down to manageable levels. The nation is recording over 150,000 new infections per day, as the overall death toll approaches a quarter million in the U.S.
New York City said its schools would revert to online learning again, as the city’s test-positivity rate reached 3%, raising fears it was on the brink of another wave after getting slammed last spring. Parents grumbled that other establishments were still open even as their kids lose out on in-person learning.
In better news, the FDA approved the first COVID-19 test that can be fully completed at home. The handheld test from Lucira Health spits out results from a nasal swab within 30 minutes, although it can only be acquired with a prescription and likely won’t be widely available for online order until spring.
Health Secretary Alex Azar told Americans to wear masks, maintain their physical distance from others and wash their hands while they wait for promising solutions, namely the vaccines.
“Even as we face daunting epidemiological trends around the country, we have reason for optimism,” he said. “Right now is not the time for anyone to let their guard down.”
Officials said they’re working through the thorny logistics of delivering the vaccines, including ways for hospitals, clinics and chain pharmacies to pick the best storage options for their distribution plans.
The Moderna shots can be refrigerated for up to a month, compared to five days for the Pfizer shots, which for longer storage must be kept at minus-94 degrees Fahrenheit in ultra-low freezers or packed in dry ice within proprietary “thermal shippers” created by the company.
Operation Warp Speed officials said distributors will be well-trained in how to manage the dry ice and they expect minimum waste of doses overall, as areas figure out how to distribute doses before they degrade.
Health workers will be first in line for the vaccines, as states finalize their distribution plans, followed by other essential workers, seniors and people with high-risk conditions. The general public should get a crack at immunization by April, according to federal timelines.
Rolling out a vaccine for a brand-new disease in less than a year is unprecedented.
Mr. Slaoui, a former pharmaceutical executive, said the record pace was made possible by the creation of flexible new platforms that allow scientists to input a pathogen’s genetic information.
He likened it to a cassette player that received a tape of the new coronavirus’s material and “played the right music.”
Mr. Slaoui also appealed to people to volunteer for ongoing vaccine trials.
He said they’d be combating the pandemic and giving themselves a 50% chance of obtaining a promising vaccine (as opposed to placebo) before it is approved for general use, so there are benefits “both from altruistic standpoint and personal standpoint.”
• Tom Howell Jr. can be reached at thowell@washingtontimes.com.



Please read our comment policy before commenting.