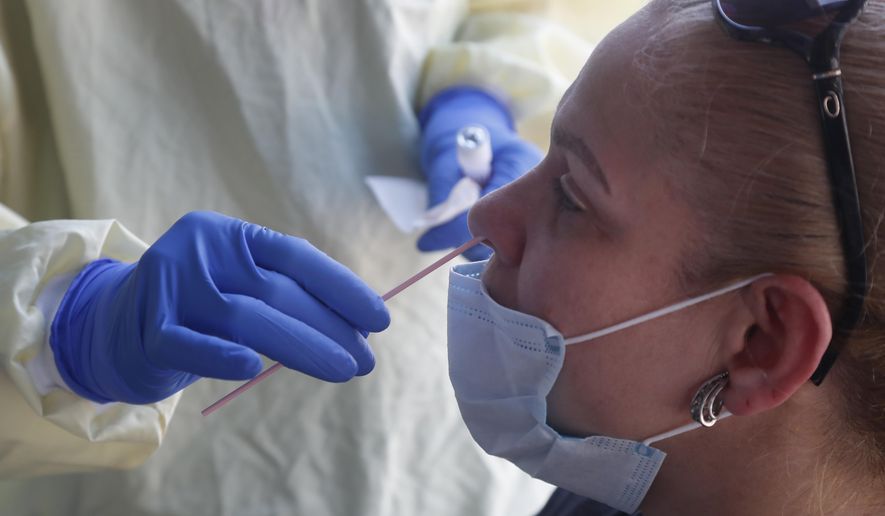
The Food and Drug Administration on Thursday approved a test to detect and differentiate viruses that cause flu and COVID-19 in people suspected of having the coronavirus.
The diagnostic test is the third to receive emergency use authorization from the FDA ahead of flu season.
“With the authorization of these tests, the FDA is helping address concerns in anticipation of this upcoming flu season during the COVID-19 pandemic, which might be especially worrying for some Americans” FDA Commissioner Stephen M. Hahn said in a statement Thursday evening.
“With just one swab or sample, combination tests can be used to get answers to Americans faster. This efficiency can go a long way to providing timely information for those sick with an unknown respiratory ailment.”
The test is an influenza SARS-CoV-2 multiplex assay developed by the Centers for Disease Control and Prevention.
The combination tests use a single sample from a patient to search for multiple respiratory diseases. Since the flu and COVID-19 can show similar symptoms, the tests could assist healthcare providers distinguish between the two viral infections while requiring fewer supplies such as swabs and personal protective equipment.
The FDA is calling on manufacturers to develop combination tests that could help preserve testing resources ahead of the upcoming flu season as the COVID-19 pandemic rages on. Almost 2.8 million Americans have tested positive and nearly 129,000 have died from the coronavirus, which is spiking in many states.
The FDA previously granted emergency approvals to BioFire Diagnostics LLC and QIAGEN GmbH for their tests, which detect other respiratory organisms with viruses that cause the flu and COVID-19.
• Shen Wu Tan can be reached at stan@washingtontimes.com.



Please read our comment policy before commenting.