Second of three parts
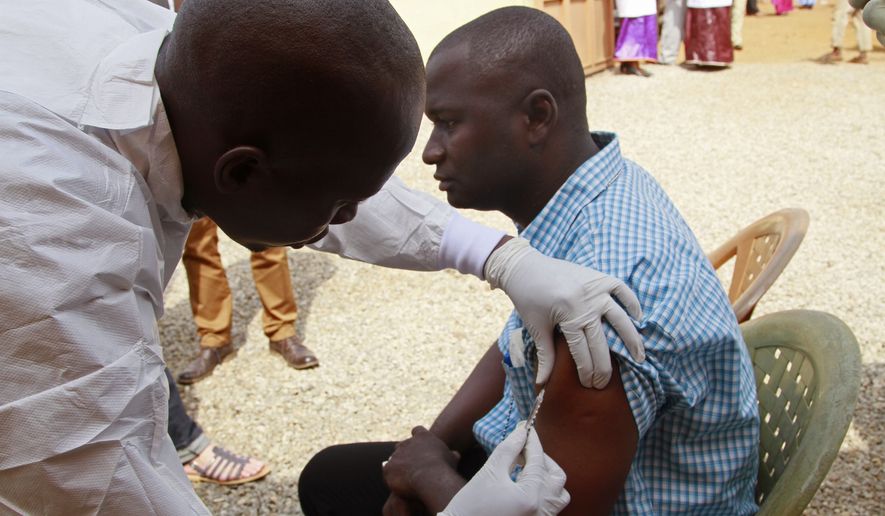
With scientists hopeful they are closing in on a vaccine to stop Ebola, policymakers are beginning to grapple with the other big questions — how much will be needed and who should get vaccinated in order to stem the next outbreak.
It’s unlikely an Ebola vaccine will join chickenpox, measles and tetanus shots as standard for the general population, but officials will have to decide how much of the vaccine to stockpile, and whether to require mandatory vaccination for those with high-risk jobs.
“Now that so much progress has been made in such a short period of time, it’s important to have those discussions,” said Dr. Mark Feinberg, vice president for vaccines at Merck, the pharmaceutical giant behind the most promising vaccine, known as rVSV-ZEBOV.
That vaccine has proven to be 100 percent effective in early rounds of a study in Guinea, guided by the World Health Organization. Other trials are going on in Liberia and Sierra Leone, the other two nations hardest hit by the 2014 outbreak.
“The one thing that’s clear to me is that we will be vaccinating lab workers who work with Ebola,” said Dr. Cliff Lane of the National Institutes of Health, co-principal investigator on the vaccine trial in Liberia.
SEE ALSO: Ebola vaccine studies progress as marketing quells suspicions of modern medicine
Amesh Adalja, a senior associate at the University of Pittsburgh Center for Health Security, said the U.S. might vaccinate certain members of the military and health workers in Ebola “hot spot countries,” but not Americans at large.
“Understanding how much will be needed will be difficult as traditional Ebola outbreaks have been much smaller historically than the West African outbreak,” he said. “Likely modeling studies and prior outbreak sizes will inform the effort.”
Past epidemics have exposed supply chain problems. But the World Health Organization says Merck has offered assurances that it can ramp up production if needed, and the global vaccine alliance known as GAVI has earmarked $300 million for the purchase of several million doses.
The compound rVSV-ZEBOV was singled out because it’s one of the two most advanced vaccines under development today and showed promise in primate testing. The other, developed by GlaxoSmithKine, uses a chimpanzee cold virus and is part of the Liberia study.
Dr. Feinberg said the rVSV-ZEBOV vaccine, a single injection into the arm, was developed by the Public Health Agency in Canada during the early 2000s. Yet back then, there wasn’t much momentum behind developing the vaccine.
“This was at a time that people couldn’t imagine [an] Ebola outbreak could be as bad as the current outbreak has been,” he said.
Until the most recent epidemic, Ebola had been deemed responsible for 1,548 confirmed deaths since its discovery in 1976, according to data from the Centers for Disease Control and Prevention.
But the new epidemic, which began in December 2013, has killed more than 11,000 people in West Africa.
It is thought to be under control at this point, with only a handful of new cases reported in Guinea and zero reported cases in Sierra Leone over the past two weeks.
Earlier this month, the WHO announced that among rings of Ebola patients’ contacts — and contacts of those contacts — not a single person in Guinea who received an immediate dose of the rVSV-ZEBOV vaccine developed Ebola after the 10-day cutoff for developing immunity from the injection.
Other clusters that didn’t get the vaccine right away did see cases of Ebola, however, suggesting that the vaccine did work in stopping cases in the first group. Those findings led researchers to drop their randomization, meaning new clusters of patients’ contacts will get the vaccine immediately, and to use the same method to isolate and stamp cases across the border in Sierra Leone.
“Mass vaccination is not necessary at this stage of the epidemic,” WHO spokesman Tarik Jasarevic said. “Once more data have been collected on the vaccine, countries will need to decide whether they want to license it nationally and stockpile it for future use.”
Dr. Feinberg said Merck is in discussions with the U.S. Food and Drug Administration and other regulatory bodies about the array of data they will need to make a decision about licensing the vaccine.
While the process can often take up to a year or more, the outbreak created a sense of urgency that could speed things up.
In the meantime, the WHO and its local partners are analyzing the results of their so-far successful trial in Guinea.
The WHO website says while it is difficult to anticipate the exact date its final results will be ready, it hopes to have them by the last quarter of this year.
• Tom Howell Jr. can be reached at thowell@washingtontimes.com.



Please read our comment policy before commenting.