First of three parts
Ebola had just killed thousands of people in Liberia, and the U.S.-backed pitch to sign locals up for a vaccine trial must have seemed like a tough sell: Take part of the very virus that had devastated the region, combine it with a separate, harmless virus, and inject it into volunteers with the hope they develop Ebola antibodies.
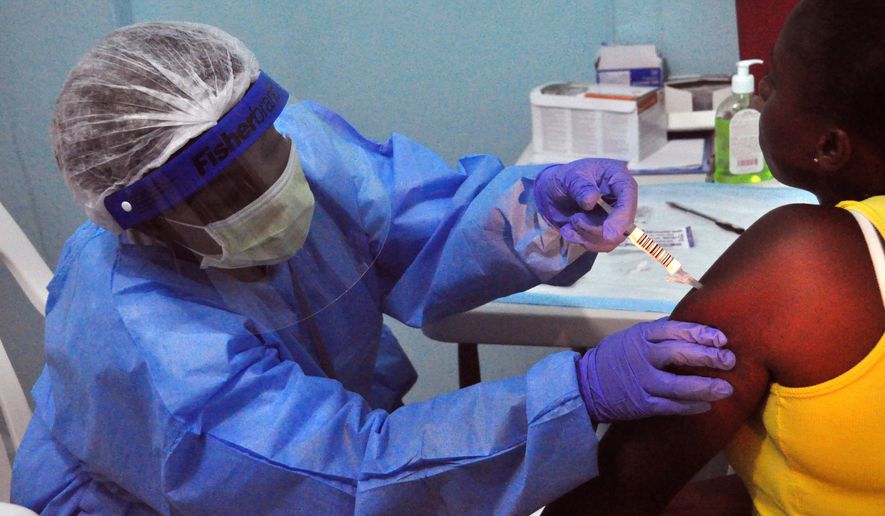
But some creative marketing, brutal honesty and good will built up from months of lifesaving humanitarian efforts paid off, with more than 1,500 volunteers now taking part in a vaccine study run out of Redemption Hospital in Monrovia, the capital.
“It was much easier than I thought it was going to be,” said Dr. Cliff Lane of the National Institutes of Health and co-principal investigator on the PREVAIL trial, a partnership between the U.S. and Liberia’s Ministry of Health. “We had support from the government. We had support from the U.S. government. We had support from the community who really understood this was important.”
A year after the Ebola epidemic swept through West Africa, claiming more than 11,000 lives and spreading panic across the globe, the disease’s spread has slowed dramatically.
Officials reported only two cases in Guinea and one case in Sierra Leone for the week ending Wednesday. Neighboring Liberia was declared Ebola-free on May 9, although several new cases in early summer put the country on high alert.
Fear of another outbreak remains high, and health officials are scrambling to try to develop a vaccine in advance of another outbreak, hoping to take advantage of changing attitudes that have made it possible to find volunteers in each of the three most-affected countries.
Each of the major studies is using a vaccine, known in lab-speak as rVSV-ZEBOV, that was developed by the Public Health Agency of Canada and licensed to NewLink Genetics Corp., which collaborated with pharmaceutical giant Merck.
Early returns from Guinea’s vaccine test are promising, though very preliminary.
But the ability to get the trials up and running is already a victory, the scientists say.
In Sierra Leone, researchers from the Centers for Disease Control signed up more than 8,000 front-line Ebola workers — from nurses to burial crews — to test the vaccine.
The CDC team noticed early on that the term “Ebola vaccine” sounded scary, so from then on it was an “Ebola-prevention vaccine.”
But they were candid about what might or might not happen.
“We worked hard to not overpromise. We said we don’t know if this vaccine works or not,” said Dr. Anne Schuchat, director of the National Center for Immunization and Respiratory Diseases at the CDC, which ran dozens of meetings in five districts throughout the country.
In Guinea, where the World Health Organization is running a vaccine trial, participants are not paid for their trouble. That actually drew people in, because the mere notion of payment tends to arouse suspicion, said Dr. Marie-Paule Kieny, an assistant director-general at WHO.
For Liberians it was important that scientists cared about their input and that nothing would be forced on them.
“Helping them understand that what we were doing was research, not clinical care, and that participation was entirely voluntary was very important,” Dr. Lane said.
It’s an amazing turnaround from early in the epidemic, when Western health workers faced skepticism and even violence in remote areas, where villagers had seen loved ones scooped up by outsiders in an ambulance and never saw them again. Burial teams with full-body outfits and sprayers looked alien to them. Some even thought Westerners were pumping Ebola into their wells, or that local governments were trumping up the virus as a ploy for international aid.
Piet deVries, who works for Global Communities, an aid group aligned with the U.S. Agency for International Development — which shifted its efforts from water and sanitation projects to the Ebola fight — said when he went to River Cess in Liberia in October, his lodgings were stoned.
“There were all kinds of rumors. It’s a country where rumor is one of the biggest ways communication happens,” said Brett Sedgewick, an adviser for Global Communities.
To counter resistance, workers set up lines of contact between traditional leaders and county health officers — channels that hadn’t existed before.
In Guinea officials tapped into Africans traditions — no pamphlets or flashy advertising — and “talked, talked, talked” to religious leaders and imams about the study, Dr. Kieny said.
Only 10 communities out of 100 refused the vaccine teams and, in gathering consent from individual enrollees, 70 percent of them agreed to participate.
Two weeks ago, the WHO announced quite promising, though preliminary, results from its trial, which tracked clusters of Ebola patients’ contacts and contacts of those contacts — a strategy that forms a protective “ring” around at-risk populations and worked to eradicate smallpox in the 1970s.
Forty-eight of the ring clusters were randomly selected to get the vaccine immediately, while 42 were vaccinated 21 days later.
While researchers found 16 cases of Ebola among the delayed group, not a single contact who received an immediate dose of the vaccine — a single injection into the arm — developed Ebola after 10 days, the study’s cutoff for developing immunity from the injection, according to findings published July 31 in the prestigious medical journal The Lancet.
“We have to see — we could still have a vaccinated person who has a case,” Dr. Kieny said. “But for the time being, it is still zero.”
While each of the trials in the three countries is testing the same vaccine, they are filling different niches.
The Sierra Leone trial will examine the efficacy of the vaccine over time in health care and other front-line workers — some are given the vaccine immediately and some six months later — while the Liberian effort is a “classical” approach to clinical research with a control group given a placebo with no therapeutic effect, meaning it is a “double-blind” trial in which neither volunteers nor staff know whether a vaccine or placebo was given.
The Liberia trial is also using a second vaccine, developed by NIH scientists and the GlaxoSmithKline company, that employs a chimpanzee-derived cold virus.
Dr. Lane said earning the backing of the host country — the Liberians kicked off the study with a formal invite to the U.S. Department of Health and Human Services — and local ambassadors helped “enormously,” as researchers engaged with residents at town hall-style meetings.
“That’s not to say there wasn’t criticism or complaints,” he added. “The media there was like the media here.”
Sometimes a hiccup amounted to little more than misplaced lingo. In a country where temperatures rarely dip below 70 degrees, Dr. Lane found himself referring to the piece of the virus taken from Ebola’s outer coat, which is used in the vaccine, as taken from the “shirt.”
“In Liberia,” he said, “there aren’t a lot of people wearing coats.”
• Tom Howell Jr. can be reached at thowell@washingtontimes.com.



Please read our comment policy before commenting.